New Study Finds U.S. Manufacturers Operating at Half Capacity
WASHINGTON, D.C., November 22, 2022 – New research shows that the United States has the manufacturing capacity to make the nation’s most essential drugs—yet most of the production lines are sitting idle.
The Center for Analytics and Business Insights, at Olin Business School at Washington University, recently released a survey on the untapped potential of U.S. pharmaceutical manufacturing.
“The 37 U.S. generic pharmaceutical manufacturing sites surveyed for this study have a total production capacity of 60.31 billion doses, defined as an oral solid (capsule/tablet) or injection, that a patient would take as a daily treatment for their medical condition,” Anthony Sardella, author of the Washington University report, senior research advisor and adjunct lecturer, said. “On an annual basis, overall, these sites are producing at just half of their production capacity, with an aggregate excess capacity of nearly 50%.”
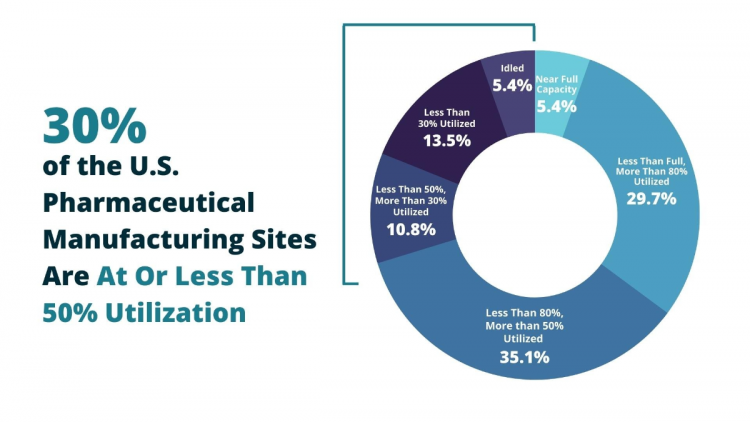
“On an annual basis, overall, these [domestic] sites are producing at just half of their production capacity…”
Anthony Sardella, Senior Research Advisor, Washington University
“On a broader scale, this study shows that American manufacturers can make it here,” said Derek Naten, Vice President of Government Affairs & Patient Advocacy at Mallinckrodt Pharmaceuticals. “With the right policies, American manufacturers could address patients’ needs across the spectrum of essential medicines and therapeutics.”
“We need consistent policy, government contracting, and industry partnerships to fulfill our domestic capacity,” said David Sanders, Executive Director of the Securing America’s Medicines and Supply (SAMS) Coalition.
See the study here: https://olinblog.wustl.edu/2022/09/olin-research-shows-us-does-indeed-have-capacity-to-manufacture-essential-drugs%EF%BF%BC/
About Securing America’s Medicines and Supply
Securing America’s Medicines and Supply (SAMS) is a multi-industry coalition of companies with the mission to strengthen the security of the medical supply chain in the United States. SAMS seeks to drive the implementation of legislation and regulation to reward and foster U.S.-made manufacturing of important pharmaceutical products, devices, and supplies. SAMS supports U.S. patients, domestic healthcare security, and U.S. jobs.