An Urgent Call to Revive the U.S. Pharmaceuticals Supply Chain

Robert J. Bowman, Supply Chain Brain
August 7, 2023
Congress is finally acting to address the severe shortage of critical pharmaceutical drugs, a crisis long in the making that was laid bare by the COVID-19 pandemic.
In May, the House Subcommittee on Oversight and Investigations took testimony from industry experts about the root causes of drug shortages in the U.S. Calling the situation “disastrous,” the panel vowed to uncover structural vulnerabilities in pharmaceutical supply chains.
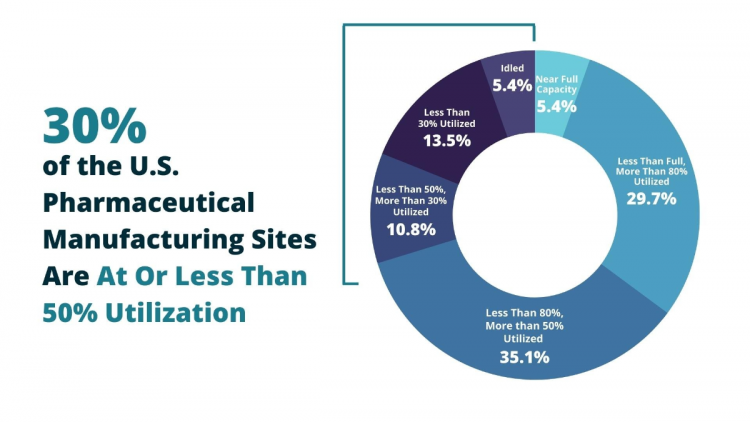
Among those testifying was Anthony Sardella, chair of the API Innovation Center and senior research adviser for the Center for Analytics and Business Insights at the Washington University in St. Louis. He cited recent research from the center which found that 30% of U.S. generic pharmaceutical manufacturing sites are operating at less than 50% utilization — at a time of crying need for the products they make. In fact, just two of the 37 sites surveyed were producing at full capacity.
The pharmaceutical industry coalition Securing America’s Medicines and Supply (SAMS) identifies 301 drugs that are currently in shortage. The group was formed in June, 2021 by Coherus Biosciences, which recruited other pharma companies “to take advantage of the new push to recreate a more resilient medical supply chain in America,” says executive director David Sanders.
This is a way bigger problem than anybody understood
Tony Paquin, co-founder, president and chief executive officer of medical products distributor iRemedy Healthcare Inc.
He says the effort is similar to the aftermath of the 2008 contamination of Chinese-made heparin, an injectable drug used to treat heart attacks and angina. The incident and subsequent recall led to a tightening up of inspections of imported drugs by the U.S. Food and Drug Administration.
The coming of COVID-19 in 2020 and 2021 created an even worse health crisis, with millions of lives threatened by a severe shortage of masks, gloves, syringes and medications. Sanders says the pandemic has led to a “permanent reform discussion” about the U.S. medical supply chain and its ability to respond quickly in a health emergency.
During the pandemic, the National Institutes of Health was forced to halt, or experienced negative impacts to, 170 cancer clinical trials, Sanders says. Almost all of the drugs involved were made in India, but lockdowns prevented FDA staff from traveling to that country to monitor production quality.
Now, with the creation of SAMS and renewed awareness by legislators of the need for reform, Sanders senses “wind in the sails.” More than half a dozen pieces of legislation have been introduced to fuel pilot programs that would allow Medicare to cover the higher cost of American-made products. And back in 2021, pharmaceuticals was among the industries targeted in an executive order by President Biden, requiring a review of critical supply chains with the goal of increasing domestic manufacturing sources.
….